

07/03/2017
© 2017 Kosmas Prassides
Inserting alkali metals into hydrocarbons containing multiple aromatic rings has long been touted as a promising way to make high-temperature superconductors, but issues with their characterization have prevented this from being confirmed. AIMR researchers and their international collaborators have now developed two complementary routes for synthesizing these materials, allowing them to be fully scrutinized for the first time — with surprising results1,2.
“Previously, it was claimed that because an alkali metal and a polyaromatic hydrocarbon had been heated together at high temperatures, the resulting black solid was an ionic salt. Since no one was able to isolate phase-pure materials, it wasn’t possible to determine the stoichiometry, structure, etc., and no property was ever authenticated,” explains team leader Kosmas Prassides from the AIMR at Tohoku University. “The previous high-temperature routes produced complex mixtures due to the decomposition of polyaromatic hydrocarbons molecules,” he adds.
The two soft-chemistry methods designed by his team avoid destroying the polyaromatic hydrocarbons. The first involves a low-temperature reduction in a solution to produce two cesium salts of phenanthrene: Cs(C14H10) and Cs2(C14H10). The second is a solid-state route using a redox-controlled reducing agent to make two potassium–C22H14 structures: K2picene and K2pentacene.
The team obtained crystal structures for all four polyaromatic hydrocarbons. “No experimentally determined crystal structures had previously been reported for any member of this class of materials,” notes Prassides.
When the team assessed the properties of the polyaromatic hydrocarbons, they were surprised to discover that none of them were superconductors.
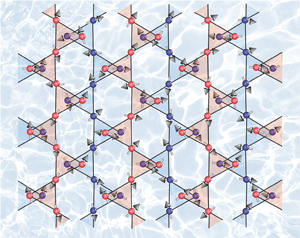
Even more unexpectedly, one of the materials, Cs(C14H10), turned out to be a candidate quantum spin liquid (see image) — a state of matter proposed over four decades ago but that had not been experimentally realized until now. “The spins in a quantum spin liquid never order — they continue to fluctuate rapidly even at a temperature of absolute zero,” says Prassides. “Each individual spin points simultaneously along an infinite number of directions and is highly entangled with other spins. As such, quantum spin liquids are predicted to host many exotic phenomena of fundamental and technological interest.” Potential applications include data storage for quantum computers.
“Cs(C14H10) adopts a complex magnetically frustrated topology and provides a rare example of a candidate quantum spin liquid, the first one arising from carbon π-electrons,” Prassides says.
Since there is a vast number of polyaromatic hydrocarbons, these routes will allow access to a compositionally, structurally and electronically diverse class of materials, notes Prassides. “We are currently synthesizing more of them and exploring their conduction and magnetic properties at temperatures close to absolute zero.”
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.