

08/25/2014
© 2014 Royal Society of Chemistry
Ionic liquids are environmentally friendly molten salts whose properties can be fine-tuned by carefully selecting their composite anion and cation molecules. In some cases, they exhibit extremely low friction when confined between two surfaces, making them candidates for replacing conventional lubricants in many applications. To advance their development and commercialization, it is vital to understand the molecular origin of their lubrication properties.
Now, Filippo Federici and colleagues from the AIMR at Tohoku University have theoretically investigated the behavior of the molecules in ionic liquids confined between two silica surfaces1. “Our study explores the relationship between the molecular size and shape of the lubricant, its interaction with the surface and its frictional response under nanoconfinement,” explains Federici.
The team simulated the behaviors of two ionic liquids containing the cation 1-butyl-3-methylimidazolium (BMIM). In one liquid, BMIM was paired with the comparably sized anion bis(trifluoromethanesulphonyl) amide (NTF2), whereas the other ionic liquid contained the much smaller anion tetrafluoroborate (BF4). Both simulations used neutral crystalline silica surfaces with protruding OH groups to confine the liquids.
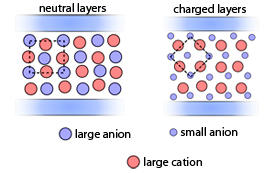
“The two liquids responded to the same silica surface in very different ways,” notes Federici. In particular, the anion size is critical in determining how the liquid molecules arranged themselves against the surfaces. As NTF2 is too large to fit between silica’s OH groups, the ionic liquid containing BMIM and NTF2 forms neutral layers containing equal numbers of anions and cations. In contrast, the BMIM–BF4 liquid forms a layer of BF4 anions closer to the silica surface, followed by alternating layers of cations and anions (see image).
Interestingly, this layering affects the ability of the ionic liquids to flow between the surfaces. When the BMIM–NTF2 layers flow past each other, similarly charged ions in adjacent layers face each other, making the structure unstable and eventually forcing it to rearrange. The BMIM–BF4 liquid is more stable, however, as ions of the same charge are not brought so close together.
The simulations throw light on a puzzling experimental result, namely that BMIM–NTF2, which is less viscous than BMIM–BF4 under normal conditions, becomes more viscous than it when the liquids are confined between planes separated by a few nanometers.
A more realistic model of the silica surfaces could offer even greater insight. Currently, the team simulates crystalline silica with a similar density to that of glass. However, as glass is amorphous, “its disorder may be an important factor in the structuring of ionic liquids at the interface,” explains Federici.
Federici Canova, F., Matsubara, H., Mizukami, M., Kurihara, K. & Shluger, A. L. Shear dynamics of nanoconfined ionic liquids. Physical Chemistry Chemical Physics 16, 8247–8256 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.