

11/25/2013
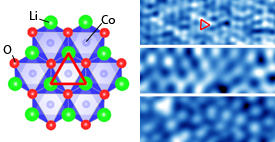
Modified from Ref. 1 © 2013 American Physical Society
Lithium (Li) ions, which are small and light, are ideal for shuttling charge between cathodes and anodes in rechargeable batteries. But despite their widespread use, researchers still struggle to understand the atomic processes behind their movements at battery interfaces. Now, Katsuya Iwaya from the AIMR at Tohoku University and collaborators have captured unprecedented images of surface atoms on lithium cobalt oxide (LiCoO2)1, an energy-rich cathode material.
In its bulk crystalline state, LiCoO2 has a structure consisting of alternating layers of lithium and cobalt oxide (CoO2). This arrangement allows lithium and cobalt atoms to undergo interactions that determine the material’s electronic activity. Understanding how these atoms arrange at cathode surfaces is difficult, however, because the behavior of lithium ions depends on the material’s composition and can elude detection with traditional microscopes.
To resolve this problem, Iwaya and his team turned to the atom-resolved imaging capabilities of scanning tunneling microscopy (STM). Normally, STM requires atomically flat surfaces to produce useful data — a challenge when investigating LiCoO2 surfaces due to their often polycrystalline and bumpy nature. The team devised a way to synthesize single crystals of LiCoO2 with a controllable lithium content that they systematically changed. Peeling these crystals apart in ultrahigh vacuum conditions produced contaminant-free, smooth surface layers.
STM experiments revealed an abundance of atomic organization patterns on the cathode surface (see image). In some areas, they saw hexagonal arrays of lithium atoms that scattered away when the researchers changed the voltage on the STM tip. Mostly though, they found cobalt oxide regions with either ordered or disordered structures. Depending on the degree of order in the cobalt oxide, the conductivity of the surface electronic states varied from insulating to metallic — a significant change from LiCoO2’s bulk crystalline properties.
By comparing the STM results to quantum chemical simulations, the researchers deduced that the unusual surface properties of their cathodes arise from non-uniform concentrations of lithium ions. In turn, this produces distinct ordering patterns that significantly modify surface electronic states. “These ordering patterns are closely related to diffusion of lithium within the crystal,” says Iwaya. “Understanding these processes in detail could lead to lithium-ion batteries with larger capacities and shorter charging times.”
Iwaya notes that these findings may also help to develop novel atomic-scale devices. “Because we can likely remove individual surface lithium ions with the STM tip, we can tune the surface electronic states at the nanoscale by controlling their ordering patterns — a potential route to very small electronic circuits.”
Iwaya, K., Ogawa, T., Minato, T., Miyoshi, K., Takeuchi, J., Kuwabara, A., Moriwake, H., Kim, Y. & Hitosugi, T. Impact of lithium-ion ordering on surface electronic states of LixCoO2. Physical Review Letters 111, 126104 (2013). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.