

07/29/2013
© 2013 Tienan Jin
Organic light-emitting field-effect transistors (OLETs) are innovative devices that combine brilliant light production with fast electronic switching. With the potential for application in flexible low-cost displays and all-organic lasers, OLET development has so far been hampered by a fundamental materials-based challenge: the molecular interactions that boost the mobility of charge carriers — a key transistor parameter — generally stifle light emission. In joint experimental1 and theoretical2 studies, a multidisciplinary research team led by Tienan Jin, Susumu Ikeda, Hiroyuki Tamura and Katsumi Tanigaki from the AIMR at Tohoku University has found a way to preserve electron mobility and generate higher luminescence efficiency in OLETs using short chains of oxygenated aromatic molecules that form unusual bent structures in the crystal state.
Previously, the researchers synthesized a promising organic semiconductor known as BP2T by linking phenyl rings and thiophene — a pentagonal aromatic molecule containing a sulfur atom — into chains called oligomers. But despite BP2T’s impressive bipolar amplification characteristics, it only converted 38% of incoming photons into luminescent emissions — a quantum yield too low for OLET applications.
The team hypothesized that substituting thiophene’s sulfur atom with oxygen, which turns the molecule into a furan ring, might enhance BP2T’s performance. As oxygen atoms are smaller than sulfur, this change should favor the growth of more tightly packed crystal structures — ideal for moving charge quickly. Furthermore, notes Jin, oligomerized furans have a strong track record of brightening luminescent materials.
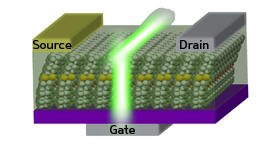
When the researchers synthesized the new furan-containing compound, BPFT, and incorporated it into an OLET (see image), they saw their strategy pay off — the luminescent quantum yield jumped significantly to 51%. Next, the team used X-ray crystallography to peer into BPFT’s molecular structure. Instead of being packed flatly like most aromatic molecules, the furan oligomers had a distinct structural kink resulting in alternation between flat and bent alignments in the solid state.
Theoretical simulations revealed that BPFT’s bending critically enhanced the OLET’s photoluminescence. According to Tamura, this particular packing arrangement ‘breaks’ the equilibrium inherent to aromatic solids, causing asymmetric electronic dipoles to form during the transition to a photo-excited state. These dipoles then generate luminescent emissions that are forbidden in totally symmetric complexes.
The researchers note that high-performance OLETs are rarely discovered because diverse expertise is required to synthesize, fabricate and model organic semiconductor devices. Collaboration between different research groups proved crucial to identifying and tailoring the BPFT structure, they add. “These results could not have been accomplished without long-term discussion and complete fusion within our team,” say Jin and Tamura.
Oniwa, K., Kanagasekaran, T., Jin, T., Akhtaruzzaman, Md., Yamamoto, Y., Tamura, H., Hamada, I., Shimotani, H., Asao, N., Ikeda, S. & Tanigaki, K. Single crystal biphenyl end-capped furan-incorporated oligomers: Influence of unusual packing structure on carrier mobility and luminescence. Journal of Materials Chemistry C 1, 4163–4170 (2013). | article
Tamura, H., Hamada, I., Shang, H., Oniwa, K., Akhtaruzzaman, Md., Jin, T., Asao, N., Yamamoto, Y., Kanagasekaran, T., Shimotani, H. et al. Theoretical analysis on the optoelectronic properties of single crystals of thiophene-furan-phenylene co-oligomers: efficient photoluminescence due to molecular bending. The Journal of Physical Chemistry C 117, 8072–8078 (2013). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.