

06/25/2012
© 2012 ACS
With their unique properties of exceptional mechanical strength, high elasticity, and resistance to mechanical wear, bulk metallic glasses hold great potential in the design of micromechanical devices and nanoscale catalysts. Although the production of nanoscale metallic glass structures is already underway, the techniques used are too expensive and time-consuming to lend themselves to mass production. Koji Nakayama, Na Chen and colleagues from the AIMR and the Institute for Materials Research at Tohoku University have now discovered a new, cost-effective technique to produce metallic glass nanowires that yields at least a few hundred million nanowires per gram of material1.
Unlike the structure of crystalline metals, metallic glasses have an amorphous and tightly-packed atomic structure, which makes them tough and resistant to mechanical deterioration. Metallic glasses are produced using a melting process, in which the molten liquid requires a high viscosity, as well as a high “spinnability” — a tendency to form elongated shapes — in order to yield nanowires.
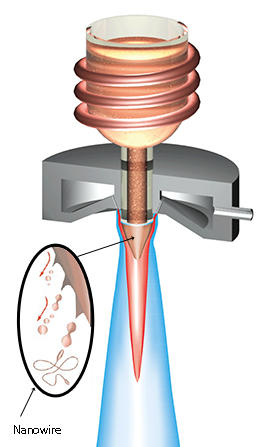
Nakayama and his team found they could create these conditions in a process called gas atomization, commonly used in the production of metallic powders. During this process, molten metal flows from the nozzle of a crucible and meets high-speed gas at the exit, which breaks up the metal stream into a variety of particulate shapes (see image). By supercooling the molten alloy below its melting point, the researchers could increase the viscosity of the melt stream prior to the gas atomization process. Though unremarkable to the naked eye, the atomized products form a metallic lump that contains a tangle of long metallic glass nanowires with diameters of 50–2,000 nanometers.
“The length to diameter of the wire (aspect ratio) increases exponentially with decreasing temperature below the melting point,” explains Nakayama, who established a “spinnability rule” to predict how nanowires made from different metallic glasses have an aspect ratio that is dependent on atomization temperature.
The team has so far focused on two metallic glasses — Zr65Cu18Ni7Al10 and Fe76Si9.6B8.4P6 — but plans to make nanowires that contain important catalytic elements like platinum and palladium. “Metallic glass nanowires will have a significant impact on catalyst research,” says Nakayama. Since nanowires have a large surface-area-to-volume ratio, they are more catalytically active per gram of material — one of the goals of green chemistry. In future, such metallic glass nanowires may also be formed from magnetic elements, which could have useful applications in the design of miniature devices with a high-frequency impedance response to a changing magnetic field.
Nakayama, K. S., Yokoyama, Y., Wada, T., Chen, N. & Inoue, A. Formation of metallic glass nanowires by gas atomization. Nano Letters 12, 2404–2407 (2012). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.