

04/30/2012
© 2012 APS
The exceptional resilience of bulk metallic glasses (BMGs) combined with their high resistance to corrosion and wear makes BMGs a highly sought-after material in mechanical applications. With a lower thermal conductivity than crystalline metals, BMGs also offer potential applications in thermal management. Divided into two general groups, BMGs are classified as either metal-metal-based glasses or metal-metalloid-based glasses. Fundamentally, however, the atomic structure of metal-metalloid-based glasses is still intriguing and remains a matter of debate among experts in the field.
In metal-metal-based glasses comprising of only metallic elements, their structure maximizes atomic packing. However, the situation is much more complex for metal-metalloid-based glasses, which also comprise atoms such as phosphorus, boron, silicon and carbon that tend to saturate their charge through covalent and coordination bonds. Because the number, direction, and length of these bonds follow specific rules, they inevitably disrupt the dense packing of the metal atoms in metal-metalloid-based glasses.
Until now, it has remained unclear how these contrasting experimental observations can be reconciled. Mingwei Chen, Pengfei Guan and colleagues from the WPI-AIMR may have now solved the puzzle by describing a hybrid between a covalent-bond mediated structure and a densely-packed icosahedron structure for a model metal-metalloid BMG, palladium–nickel–phosphorus1.
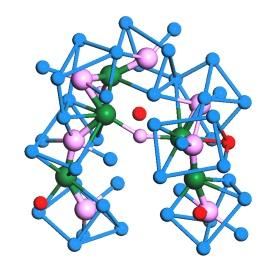
“This BMG was actually the first bulk metallic glass reported in the literature, and so far it still has the best glass-forming ability among ternary metal-metalloid alloys,” explains Chen. It was therefore a natural choice for the team to focus on unraveling the structure of palladium–nickel–phosphorus. In their analysis, the team performed both X-ray diffraction experiments and ab initio molecular dynamics simulations. The results suggest that phosphorus coordinates with both palladium and nickel, while metallic bonding occurs between these two metallic elements. The researchers propose the structure of palladium–nickel–phosphorus consists of two types of clusters perfectly interconnected, in which tri-capped trigonal prisms centered on phosphorus atoms are linked together by nickel-centered densely-packed icosahedra.
This hybrid structure, which satisfies both the necessity of charge saturation for phosphorus atoms and the dense packing requirement for metal atoms, exhibits the lowest energy achievable in the compounds and is therefore relatively disordered, yet stable.
These findings do not only explain the well-known glass-forming ability of the alloy palladium–nickel–phosphorus, but also hold a wider significance in our understanding of the formation of metal–metalloid glasses. “This may be a universal structural model for metal–metalloid glasses,” says Chen. “We have also found the same packing scheme in different alloy systems.”
Guan, P. F., Fujita, T., Hirata, A., Liu, Y. H. & Chen, M. W. Structural origins of the excellent glass-forming ability of Pd40Ni40P20. Physical Review Letters 108, 175501 (2012). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.