

11/29/2010
© 2010 APS
Many industrial processes, such as the internal combustion engines in cars, generate significant amounts of heat. If some of this heat could be converted into usable energy in the form of electricity, energy consumption could be reduced considerably. Thermoelectrics are a class of materials that convert heat to energy, and unsurprisingly, they are the subject of intensive scientific investigation.
Researchers from the WPI-AIMR at Tohoku University in collaboration with researchers from the university’s departments of physics and chemistry have now investigated how the movement of atoms in a particularly promising class of thermoelectrics called clathrates can influence their thermal properties1. “Clathrates are one of the most promising candidates for efficient thermoelectric energy conversion,” says Katsumi Tanigaki, who led the research team.
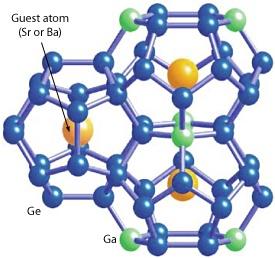
Clathrates are cage-like compounds that enclose trapped guest atoms (Fig. 1). The size of the guest atom in relation to the host structure determines how freely it can move and ‘rattle’ in its cage. These rattling motions suppress heat transmission and therefore have a strong influence on heat conductivity. As thermoelectricity is based on heat differences within a material, lower heat conductivity leads to better thermoelectric performance. The open structures of clathrates are well-suited for this purpose, particularly when the guest atom is small and therefore interacts less with the surrounding cage as it vibrates.
In their systematic study, the researchers carefully investigated two similar clathrate compounds: Sr8Ga16Ge30 (SGG), which contains a strontium guest; and Ba8Ga16Ge30 (BGG), which houses a barium guest. Strontium is smaller than barium, and consequently SGG is the better thermoelectric.
An important contribution to the heat conductivity of clathrates is the movement of the guest atom within a particular cage. To reach a good understanding of this process, it is important to distinguish the heat capacity contribution of atomic movements from that of electron transport. Through careful preparation of samples with different electron concentrations, the researchers were able to separate the influence of electrons on thermal properties from the effect of strontium and barium atomic motion.
As the origin of SGG’s excellent thermoelectric properties, the researchers identified that the off-center vibration of strontium atoms in the cage — as opposed to the centered vibration of the barium guest — leads to enhanced coupling between strontium atom movement and electrons in the crystal. This proved to be the key to understanding the enhanced thermoelectric performance of SGG, says Tanigaki. “The anharmonic movement of the guest atoms is responsible for their poor thermal conductivity, and could provide a blueprint for enhanced thermoelectric materials.”
Xu, J., Tang, J., Sato, K., Tanabe, Y., Miyasaka, H., Yamashita, M., Heguri, S. & Tanigaki, K. Low-temperature heat capacity of Sr8Ga16Ge30 and Ba8Ga16Ge30: Tunneling states and electron-phonon interaction in clathrates. Physics Review B 82, 085206 (2010). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.