

09/28/2009
Bulk metallic glasses (BMGs) are metal alloys that have been cooled such that they solidify without crystallizing to form an ordered lattice. Unlike conventional metals, they soften and flow readily at moderate temperatures, allowing them to be cast or molded into intricate shapes. This unique processability, and their high strength, wear and corrosion resistance, make these materials attractive for application in biomedical and microelectromechanical devices.
Despite being stronger and more wear-resistant than conventional metals, some metallic glasses are also brittle. Now, Na Chen from Tohoku University's Advanced Institute for Materials Research (AIMR) and collaborators1 have introduced small amounts of silicon (Si) into a metallic glass combining palladium (Pd), nickel (Ni) and phosphorus (P) to create an alloy with enhanced glass-forming ability and ductility.
Noting that an exceptionally ductile metallic glass of Pd–Si had been developed previously2, and that binary alloys of palladium or nickel with either silicon or phosphorous form similar phases on cooling, the researchers expected that the addition of silicon could also improve the ductility of the ternary Pd–Ni–P alloy without significantly affecting its glass-forming ability.
To produce their alloy, the team sealed well-defined compositions of high-purity metal powders in quartz tubes under high vacuum, and melted the mixtures homogeneously at 1,323 K. They then cooled the tubes in water to solidify the Pd–Ni–Si–P ingots. Finally, they melted the ingots and cast the alloy into 2 mm-diameter cylinders.
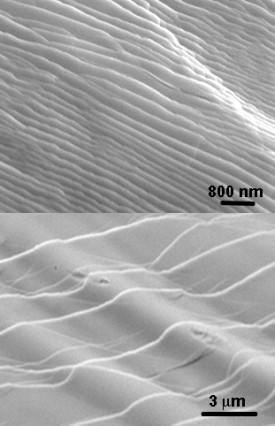
Chen and her colleagues found that, under compression, the Pd–Ni–Si–P alloy could tolerate three times as much strain as the original Pd–Ni–P alloy before breaking. The broken Pd–Ni–Si–P surfaces displayed wavy nanometer- and micrometer-sized steps (Fig. 1). “These steps are usually observed on the fracture surface of brittle materials,” explains Chen. “But in the case of the Pd–Ni–Si-P metallic glass, the nanoscale and microscale wavy steps were formed at the final stage of the fracture.”
The researchers showed that Pd–Ni–P displays two crystallization stages at different temperatures. When they added as little as 5% silicon, however, only one of those stages remained. They therefore suggest that the addition of silicon to Pd–Ni–P traps the metals in a liquid-like arrangement where the atoms bind loosely to each other.
“Compared to metallic additives, silicon is a much smaller atom, and thus has a large atomic size difference with the main constituents, palladium and nickel, which favors the formation of a non-periodic dense packing structure and suppresses crystallization,” explains Chen.
The researchers are currently focusing on developing other ductile and biocompatible BMGs and plan to use these high-performance, durable materials to manufacture small devices for microelectromechanical systems.
Chen, N., Louzguine-Luzgin, D.V., Xie, G.Q., Wada, T. & Inoue, A. Influence of minor Si addition on the glass-forming ability and mechanical properties of Pd40Ni40P20 alloy. Acta Materialia 57, 2775 (2009).
Yao, K.F., Ruan, F., Yang, Y.Q. & Chen, N. Superductile bulk metallic glass. Applied Physics Letters 88, 122106 (2006). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.